Table of Contents
- What is pH?
- The pH Scale
- Why Does Soil pH Matter?
- What Is pH Lockout?
- How to Test Your Garden pH
- What Does Garden Soil pH Indicate About Garden Health?
* Our articles never contain AI-generated slop *
If you don't regularly test your garden soil pH, you might experience what's known as nutrient lockout - when vital elements in the soil become inaccessible to your plants that need them.
We're talkin all about why you should test your garden soil pH, how to do it, and what you should know.
First, a refresher on some science some basics to lay the foundation:
Disclaimer: This post may contain affiliate links. Refer to the privacy policy for more information.
What is pH?
Technically, pH is the measure of the potential of hydrogen - that is the concentration of hydrogen atoms (H⁺) in a solution.
pH = −log10[H⁺]
In layman's terms, pH is a measure of the acidity or alkalinity of the solution.
For our purpose here we're interested in the alkalinity or acidity of soil - as this factor has a profound effect on the ability for plants to uptake nutrients and to grow.
If you're growing typical herbs and veggies in your garden, you'll likely want to shoot for a pH around 6.5. Some plants, such as blueberries, prefer more acidity than that, so it's always a good idea to identify the desired pH for what you're growing.
The pH Scale
The pH scale is typically represented as going from 0.0 to 14.0.
These are not hard limits, and some solutions can achieve a pH below 0.0 or above 14.0 - but it's not likely that you'll ever encounter a substance that falls outside of these bounds if you aren't some sort of chemist.
7.0 is exactly neutral.
0.0 is the most acidic end of the scale (though lower pH measurements are possible).
14.0 is the most alkaline end of the scale (though again, higher pH measurements are possible).
One important note about pH is that the scale is logarithmic, not linear. Each whole step number is a power of 10, relative to the previous number.
- A pH of 8.0 is 10 times more alkaline than a pH of 7.0.
- A pH of 9.0 is 100 times more alkaline than 7.0
- A pH of 10.0 is 1,000 times more alkaline than 7.0
- A pH of 11.0 is 10,000 times more alkaline than 7.0
- A pH of 12.0 is 100,000 times more alkaline than 7.0
- A pH of 13.0 is 1,000,000 times more alkaline than 7.0
- A pH of 14.0 is 10,000,000 times more alkaline than 7.0
This goes in reverse as well. 6.0 is 10x more acidic than 7.0, etc.
Since we're shooting for roughly 6.5 to grow veggies, that's 5 times more acidic than 7.0
I mention the exponential nature of this scale because it helps illustrate how small variations in pH can have an outsize effect on plant health.
Too far in either direction and your plants will begin showing signs of nutrient lockout.
Why Does Soil pH Matter?
Soil pH is a crucial component to get right, because each macro and micronutrient is only available to the plant within a certain pH range.
Join The Grower's Community
Your space to connect, learn, and belong 🌱
Check It Out!
When soil is too alkaline, many of the nutrients that your plants need will be locked out, leaving plants deficient and unable to absorb nutrients that may be readily available in your soil.
An incorrect soil pH range can therefore present itself as any number of deficiencies in your plants and leave you scratching your head as to what the problem is.
For instance, your plants may be showing chlorosis, a sign of iron deficiency even when there is enough iron in the soil for your plants to use. If your soil is too alkaline though, it will lock that iron out and prevent your plants from being able to uptake it.
This is why correcting your soil pH should be a higher priority than reacting to each deficiency that may present itself.
What Is pH Lockout?
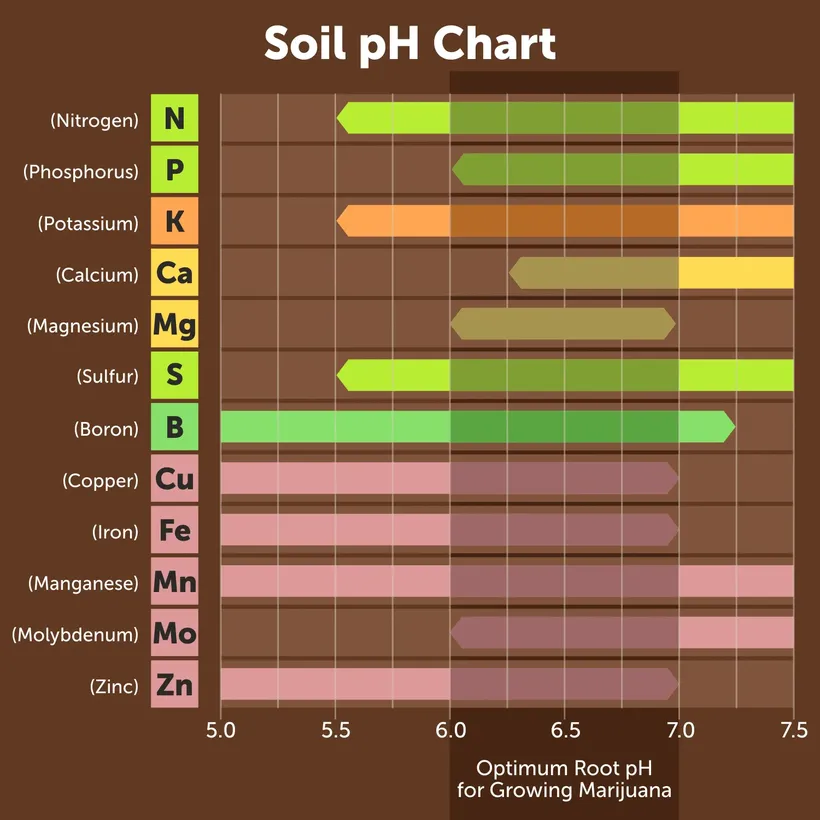
most vegetables have similar pH tolerance
For example I was working with a gardener trying to resurrect a dying peach tree which was showing yellowing leaves (chlorosis) due to an iron deficiency.
The gardener applied chelated iron to the tree, figuring that would help correct the problem.
After I ran a soil pH test, however, I found that the peach tree was standing in extremely alkaline soil with a pH above a 9.0
Iron is completely locked out of most plants when the pH rises too high. For peach trees, iron lockout begins at a pH of 7.0 and above. Clearly, the 9.0 pH would not allow any iron absorption at all.
As expected, the application of iron did nothing for the peach tree.
An application of soil sulfur, however, helped to drop the pH down to an acceptable level along with some heavy soil-building techniques to improve organic matter content and soil quality.
Dig Cool Merch?
After this, the chlorosis began to fade and the peach tree started to look a whole lot better.
This is just one example of pH lockout, but it's a very common issue especially here in the Sonoran Desert where native soil is far too alkaline to grow most vegetables in without some soil-building work.
How to Test Your Garden pH
Grab yourself a pH test kit or a pH tester pen. These are inexpensive, and are sold online, at your local hydro shop, or possibly even at garden centers.
You'll also need some pure distilled water, OR some water that's been pH-adjusted to an exactly neutral 7.0 pH. Grab a jug of distilled water at the grocery store.
The only other thing you need for a soil pH test is any small container which you can add a pinch of soil and a few ounces of water to. Any small jar or cup will work:

Grab a pinch of dirt to sample. This may be from your garden, your native soil, your potting mix, seed-starting mix, or compost.
Consider here that the depth of your soil sample does matter. Sample soil at multiple different depths to gain a better understanding of your garden and how your amendments are affecting it.
Once you've got your sample(s), put one in the jar - it doesn't have to be full, just as long as you've got enough to get a good test on.

Add your distilled water, and shake it up so you've got some nice watery mud you can test.
Then grab your digital pH meter and give it a good reading, being sure to wait at least 30 seconds, or until the pH number stops changing. Be patient here until you've got an accurate reading.
What Does Garden Soil pH Indicate About Garden Health?
A healthy soil microbiome will be able to automatically maintain a pH that is close to ideal. If your pH is very far from the ~6.5 required for veggies, you've got some work to do on your dirt.
Alkaline Soil
Here in Tucson our native soil often has a pH of 9.0+ (crazy, I know!) and is extremely alkaline. Most plants which are not adapted to the desert here are unable to tolerate such levels and will suffer from severe nutrient lockout without adjusting the soil pH.
As you build healthy soil, though, the alkalinity of the native soil is tempered by a thriving microbiome and increased organic matter. Building healthy soil alone is enough to drop the alkalinity of native desert soil tremendously. It may not end up exactly in the ideal ~6.5 pH zone, but will come far closer than 9.0+ after some time spent building soil and adding organic matter.
I still supplement with some soil sulfur twice a year, but my thriving microbiome does most of the pH adjusting automatically.
I know that if I test my soil pH and see a number that's too high, this is an indication that I need to do more work on building healthy soil, and that my soil has too much low-quality native alkaline dirt in it which needs to be diluted with more rich organic matter.
To learn more about dealing with alkaline soil, check out our Guide to Alkaline Soils.
Acidic Soil
If your soil is too acidic for veggies (below a ~6.0 pH), you'll want to increase the alkalinity to bring it within range.
Garden lime and dolomite lime are both great choices to raise your pH. Dolomite lime is similar to garden lime, but also contains magnesium - so depending on your soil magnesium content you can choose one or the other.
That's all for now, thanks for reading!
If you have any questions, comments, or would like to connect with fellow gardeners, head on over to the forum and post there.








![Black Dirt Live Again [Blue]](/media/product_images/black-dirt-live-again-[blue]_sticker_260x260.png)

![Don't Till Away Your Carbon [Taffy]](/media/product_images/dont-till-away-your-carbon-[taffy]_sticker_260x260.png)